Limited data exists on the optimal dosing weight for intravenous (IV) acyclovir in patients with a body mass index (BMI) greater than 40 kg/m^2. One retrospective study suggested that adjusted body weight (AdjBW) dosing resulted in reduced drug exposure compared to ideal body weight (IBW) dosing, while another study indicated lower acyclovir exposure in obese patients dosed on IBW compared to normal-weight patients dosed on total body weight (TBW), suggesting potential similarity in exposure ...
A poster abstract describing a 2020 retrospective observational chart review assessed the impact of different dosing strategies of intravenous (IV) acyclovir in obese patients. The study included 51 adult patients with a body mass index (BMI) greater than or equal to 30 kg/m^2 who received at least 48 hours of high-dose IV acyclovir therapy. Efficacy analysis was conducted by stratifying patients into ideal body weight (IBW), adjusted body weight (AdjBW), and total body weight (TBW) groups. Treatment failure was observed in 3 out of 51 patients (1 patient in IBW group, 2 patients in AdjBW group, p= 0.445). Median length of stay (p= 0.977) and median duration of IV therapy (p= 0.78) did not show significant differences. Nephrotoxicity occurred in 22.2%, 19.2%, and 22.7% of patients in the IBW, AdjBW, and TBW groups, respectively (p= 1). The study suggested that, while comparing different dosing modalities, there were no significant differences in the outcome of infection, duration of...
READ MORE→
A search of the published medical literature revealed
3 studies investigating the researchable question:
What information is available for dosing acyclovir IV in obese adults and pediatrics? e.g. use adjusted body weight based on BMI, weight 120-140% x IBW, or other suggestions.
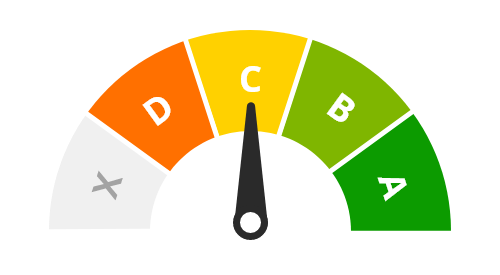
Level of evidence
C - Multiple studies with limitations or conflicting results
READ MORE→
[1] Mulvey N, Jain S, Falsetta K, Doan TL. 196. Assessing the Clinical Impact of Intravenous Acyclovir Dosing in Obese Patients: Should We Be Using Ideal, Adjusted, or Total Body Weight?. Open Forum Infect Dis. 2020;7(Suppl 1):S102-S103. Published 2020 Dec 31. doi:10.1093/ofid/ofaa439.240
[2] Smith, M. Keith, A. Abstract only. Published 2020. Accessed June 7, 2023. https://pharmacy.unc.edu/wp-content/uploads/sites/1043/2020/06/Smith-Mary.pdf
[3] Gaeta F, Conti V, Pepe A, Vajro P, Filippelli A, Mandato C. Drug dosing in children with obesity: a narrative updated review. Ital J Pediatr. 2...