Neostigmine remains the only pharmacologic agent with consistent controlled evidence and guideline endorsement for acute colonic pseudo-obstruction, with multiple trials and meta-analyses demonstrating high response rates when used after failure of conservative therapy. In contrast, pharmacologic management of adult chronic intestinal pseudo-obstruction and pediatric PIPO is based on very low–quality evidence and is largely adjunctive and individualized, with agents such as pyridostigmine, pr...
Acute colonic pseudo-obstruction (ACPO):
The 2020 American Society for Gastrointestinal Endoscopy (ASGE) guideline identifies neostigmine as the pharmacologic agent of choice for ACPO and recommends its use in patients who are not candidates for conservative therapy, who have failed conservative therapy for up to 72 hours, or who are at risk for perforation and have no contraindication to treatment. During administration, continuous cardiac and respiratory monitoring is required, with immediate access to atropine for bradycardia, and glycopyrrolate may be coadministered to reduce hypersalivation and bronchospasm. Contraindications include intestinal or urinary obstruction and hypersensitivity, and relative contraindications include bradycardia, asthma, renal insufficiency, peptic ulcer disease, recent myocardial infarction, and acidosis. A standard dose of 2 mg IV over 3 to 5 minutes is recommended, and in patients who fail an initial dose, are partial responders, or experience rec...
READ MORE→
A search of the published medical literature revealed
4 studies investigating the researchable question:
What are the best pharmacologic treatments for intestinal pseudo-obstructions?
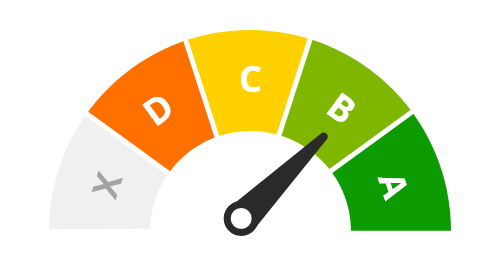
Level of evidence
B - One high-quality study or multiple studies with limitations
READ MORE→
[1] Naveed M, Jamil LH, Fujii-Lau LL, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the management of acute colonic pseudo-obstruction and colonic volvulus [published correction appears in Gastrointest Endosc. 2020 Mar;91(3):721]. Gastrointest Endosc. 2020;91(2):228-235. doi:10.1016/j.gie.2019.09.007
[2] Alavi K, Poylin V, Davids JS, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colonic Volvulus and Acute Colonic Pseudo-Obstruction. Dis Colon Rectum. 2021;64(9):1046-1057. doi:10.109...